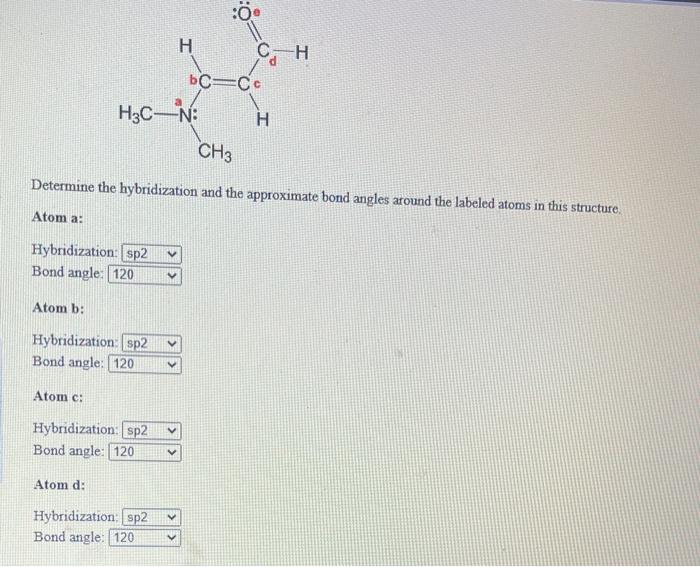
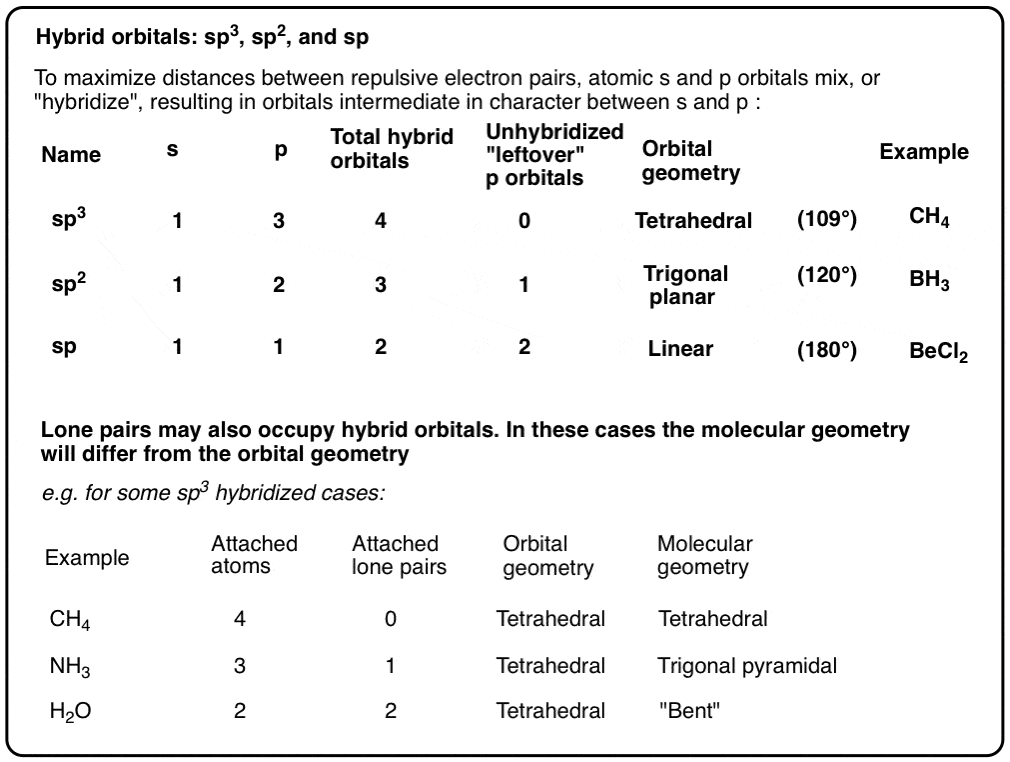
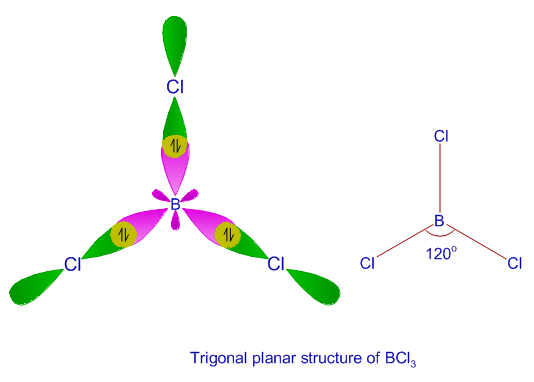
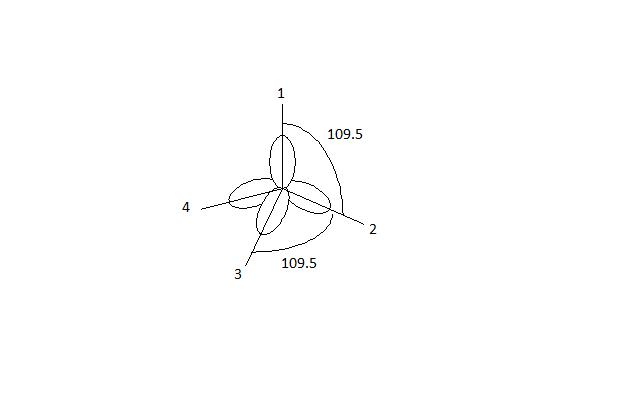
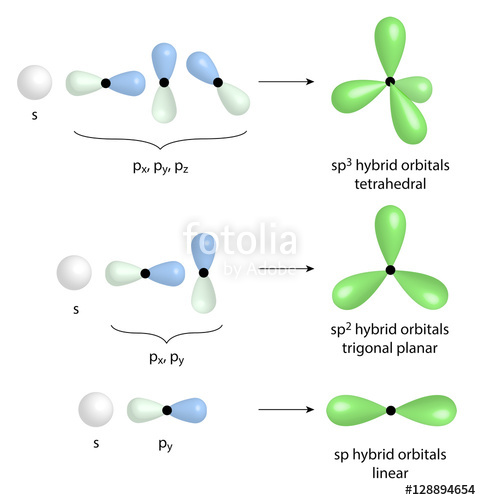
a. For the given molecule, what is the hybridization at the atom numbered 1? Enter sp2, sp3, ... b. For atom 1, what Is the bond angle? (degrees) | Homework.Study.com
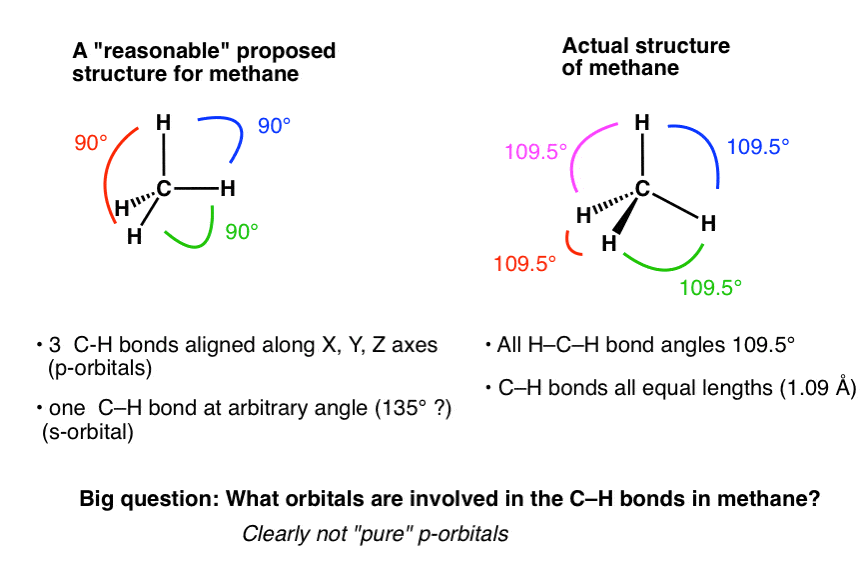
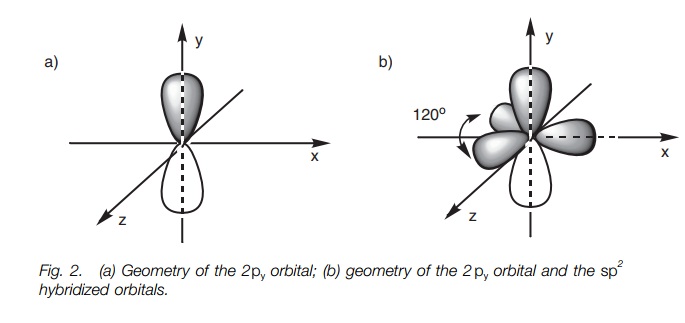
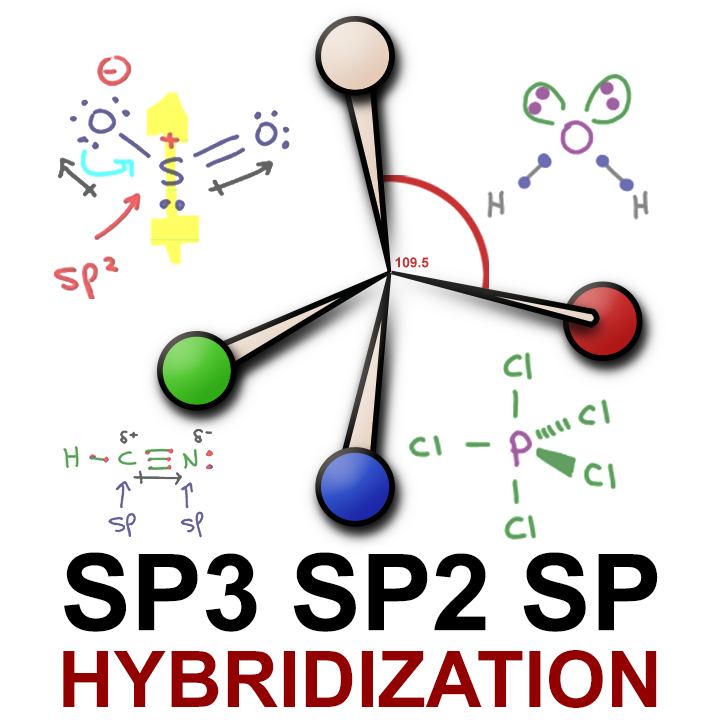
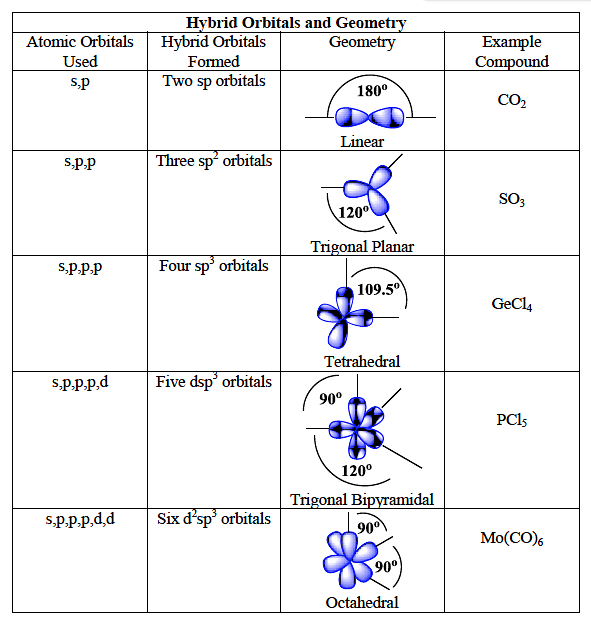
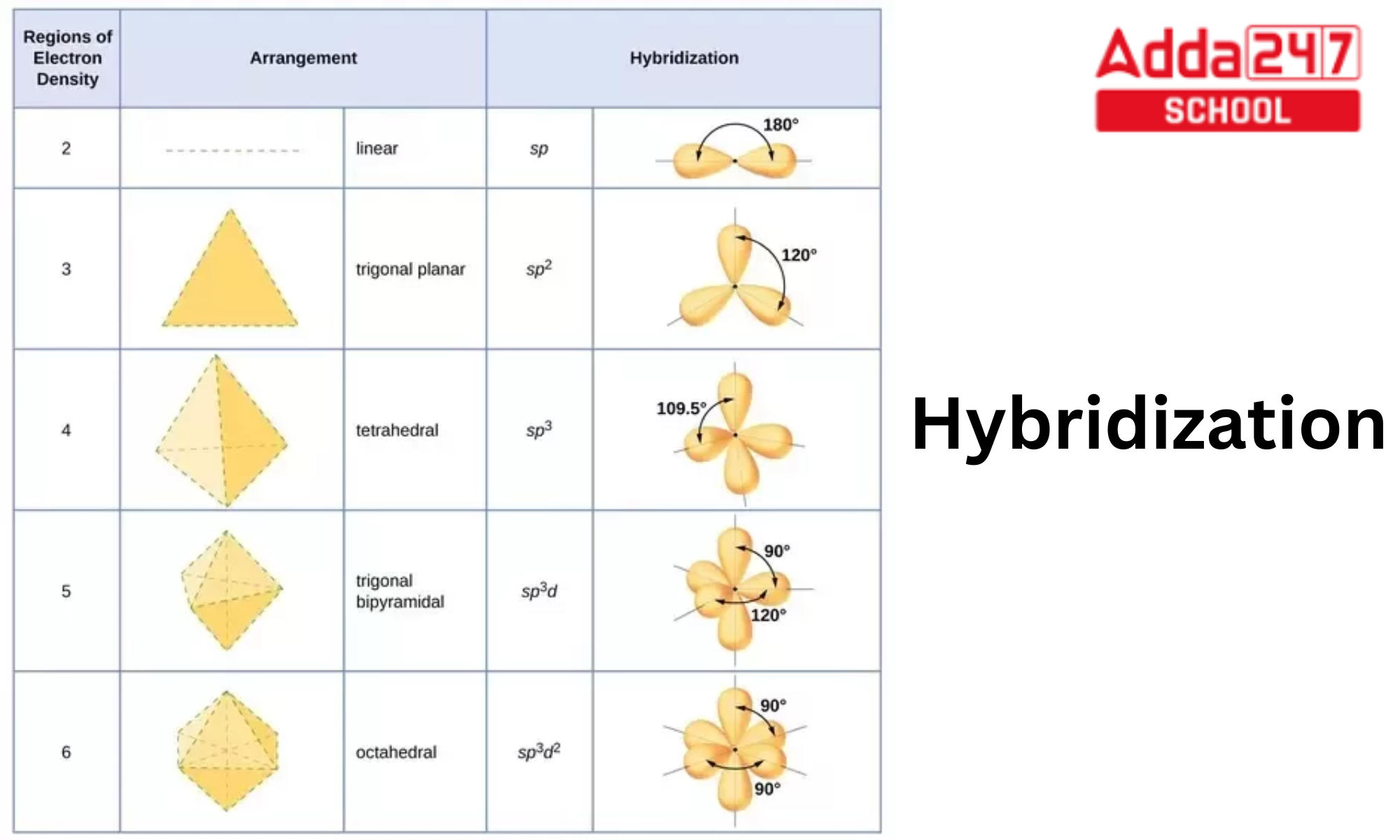
What are the shapes and bond angles of sp, sp2, sp3, sp3d, sp3d2 hybridised orbitals respectively? - Quora
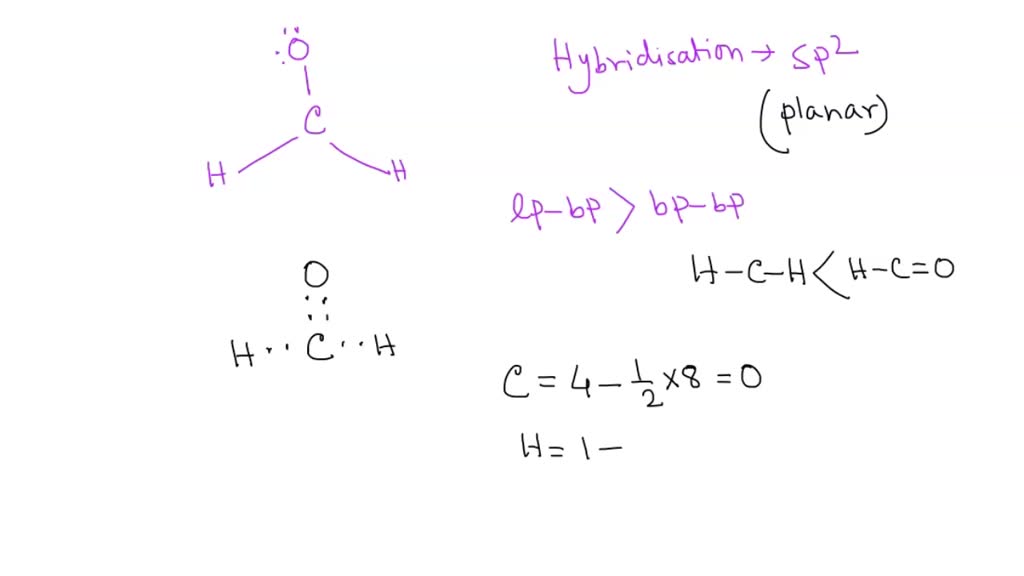
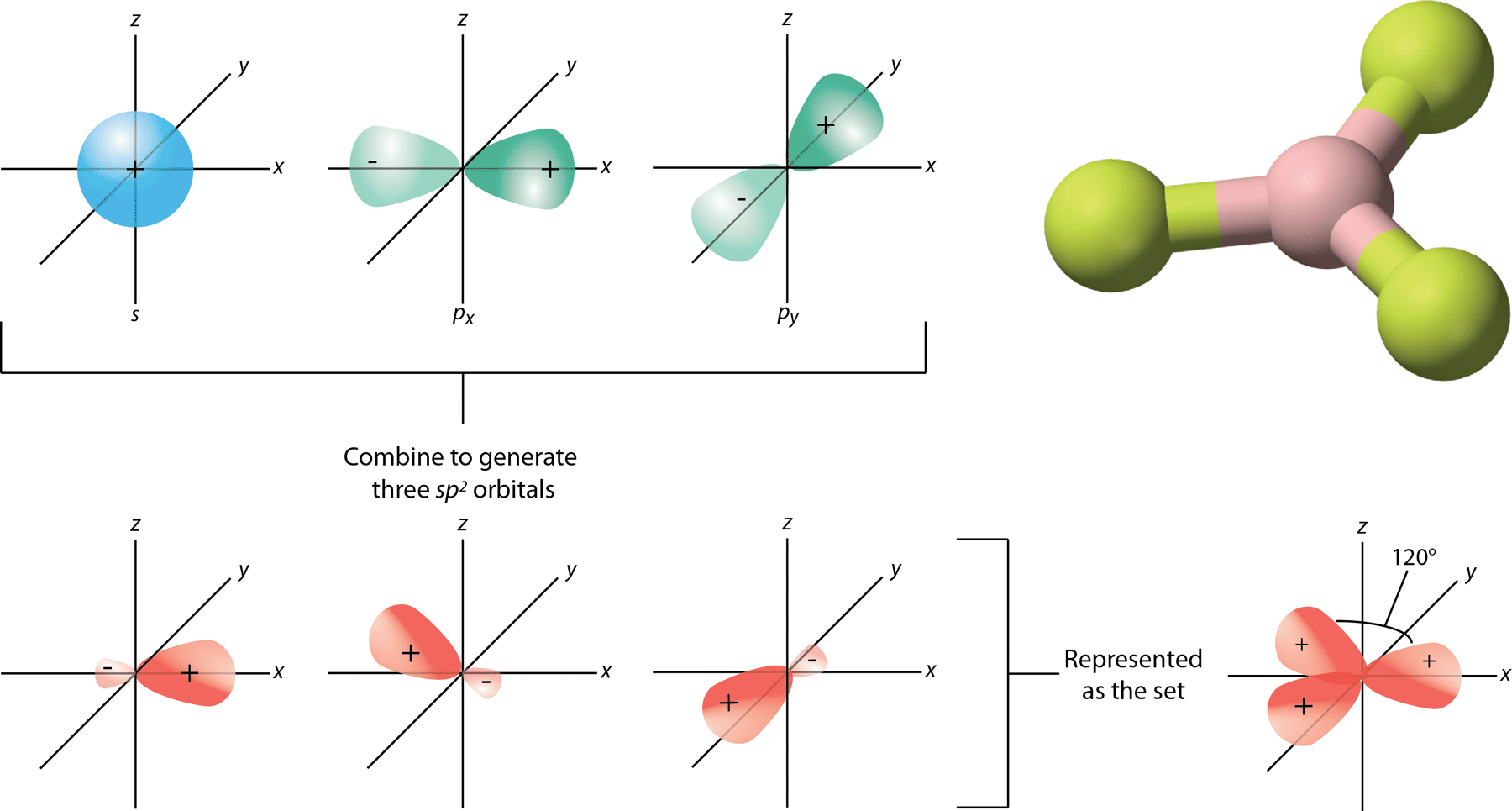
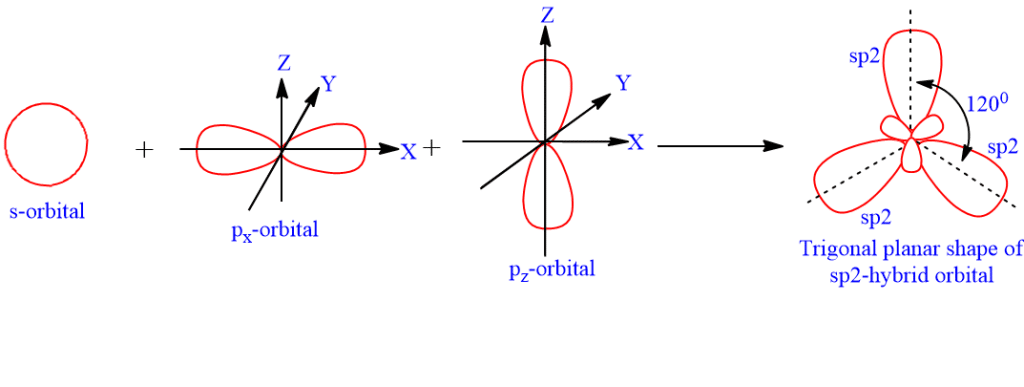
SOLVED: What is true about the CH2O molecule? Carbon utilizes sp2 hybridization. The H-C-H bond angle is expected to be slightly less than the H-C-O bond angle. The molecule contains 3 sigma

A molecule has sp2 hybridization with 1 lone pair. a. The electron pair geometry of this molecule is: b. The geometry of this molecule is: c. This molecule will have an approximate
What are the shapes and bond angles of sp, sp2, sp3, sp3d, sp3d2 hybridised orbitals respectively? - Quora