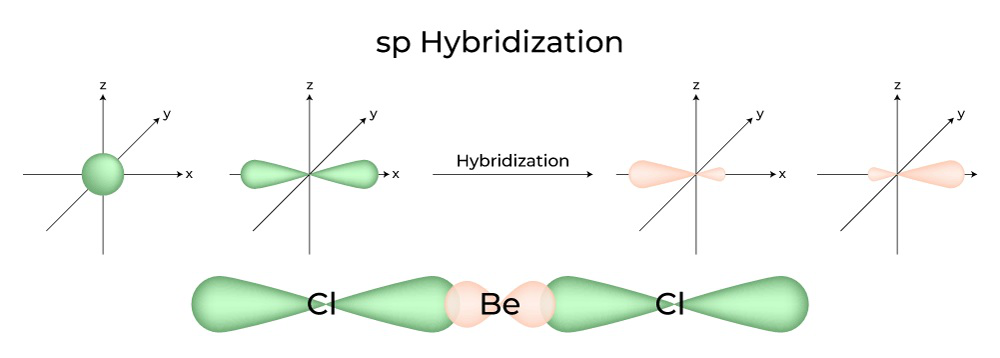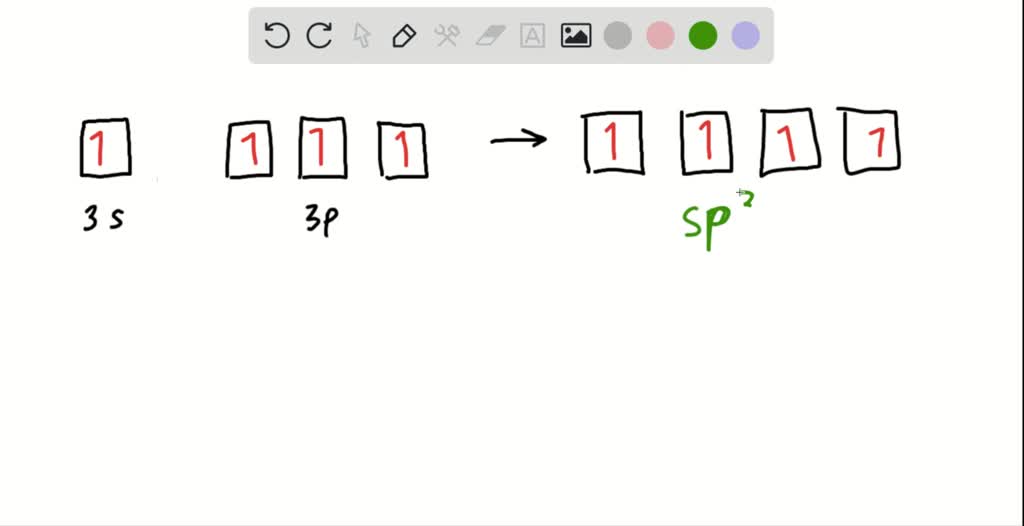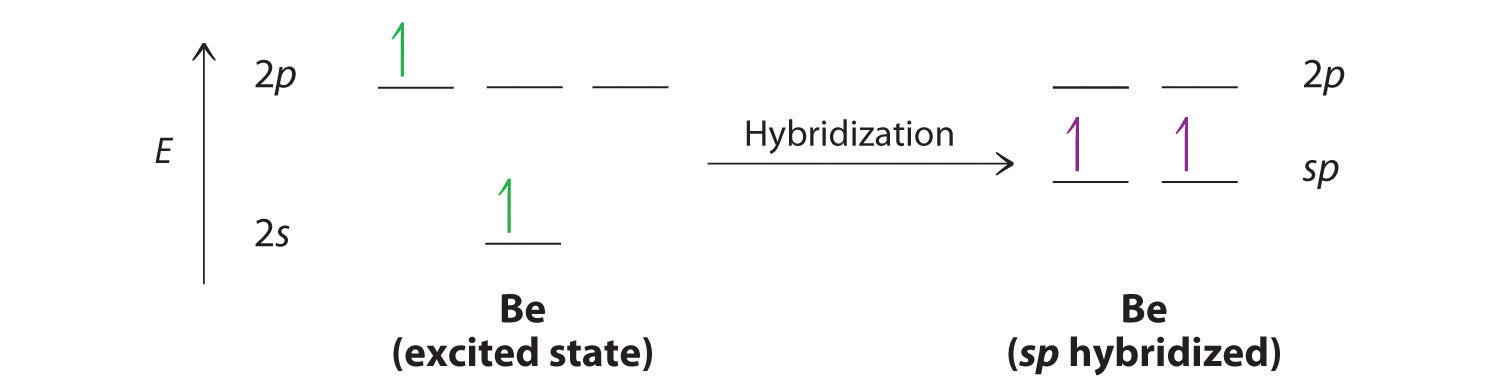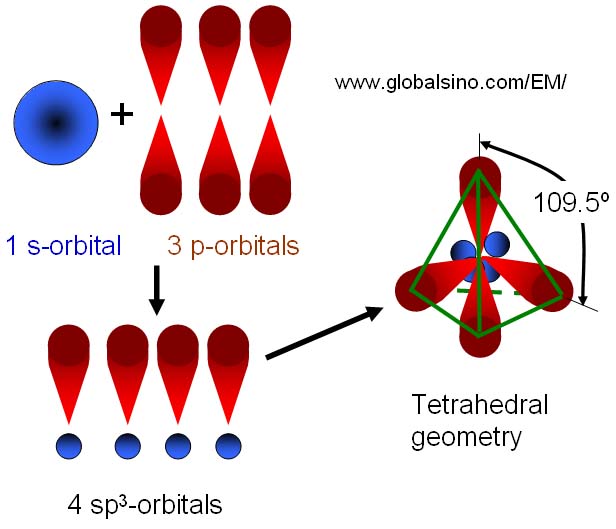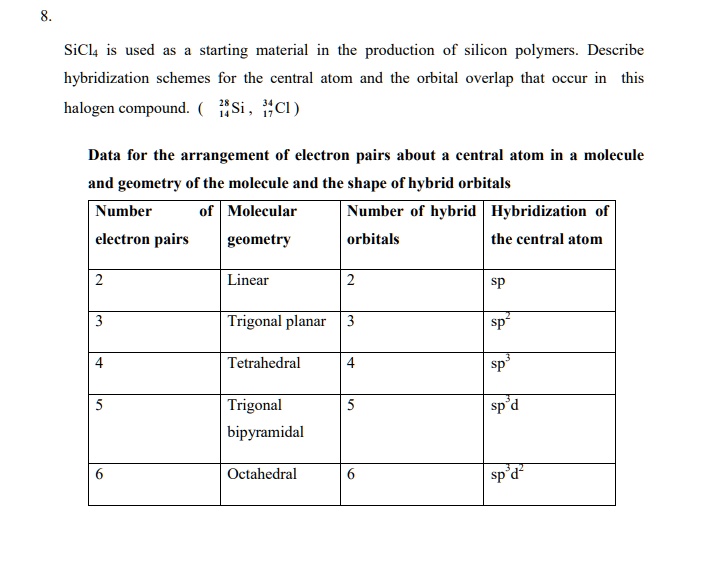
SOLVED: The production of silicon polymers is described. Hybridization schemes for the central atom and the orbital overlap that occur in this halogen compound (SiCl4) are discussed. Data for the arrangement of
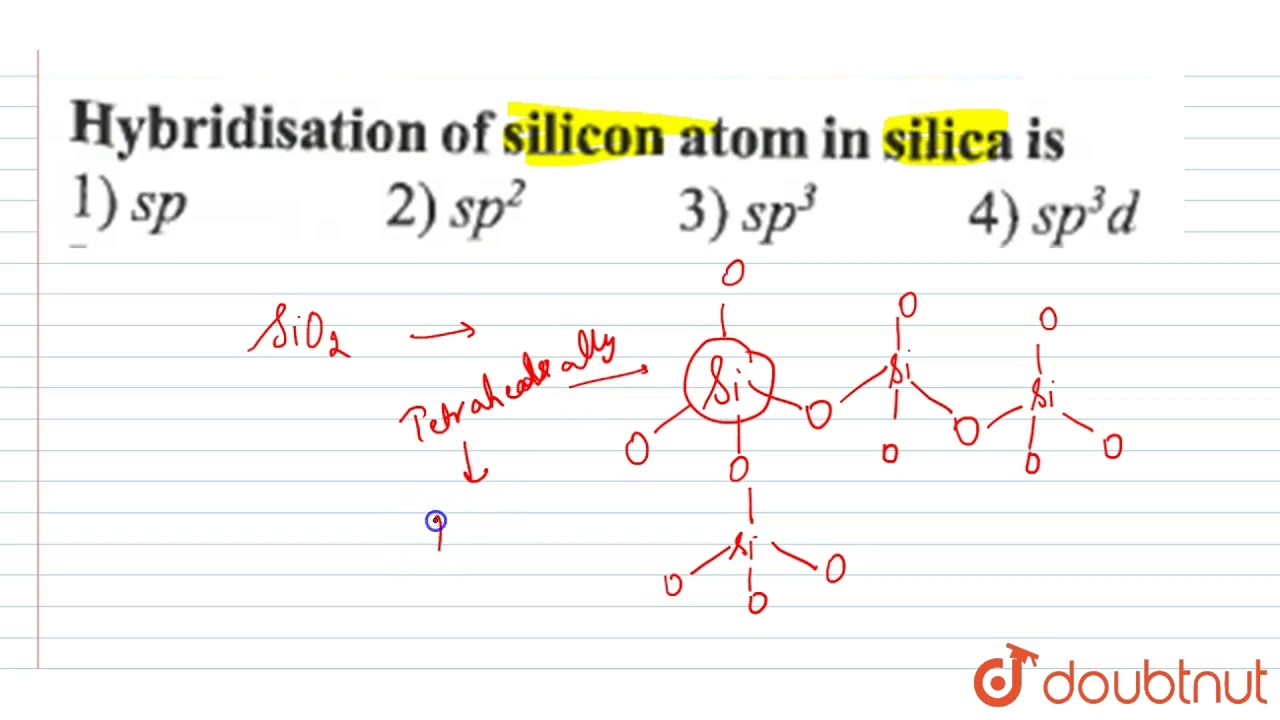
![What is the hybridization of the central atom of $SiO_2$?A.\\[sp\\]B.\\[s{p^2}\\]C.\\[s{p^3}\\]D.\\[s{p^3}d\\] What is the hybridization of the central atom of $SiO_2$?A.\\[sp\\]B.\\[s{p^2}\\]C.\\[s{p^3}\\]D.\\[s{p^3}d\\]](https://www.vedantu.com/question-sets/bfbe5949-20d8-4df4-b2df-ad4de6f9d4df3600507102077243315.png)
What is the hybridization of the central atom of $SiO_2$?A.\\[sp\\]B.\\[s{p^2}\\]C.\\[s{p^3}\\]D.\\[s{p^3}d\\]

Silicon tetrafluoride, SiF_4, is a colorless gas formed when hydrofluoric acid attacks silica (SiO_2) or glass. Describe the bonding in the SiF_4 molecule, using valence bond theory. | Homework.Study.com

a) sp 2 hybridized planar silicene (b) buckled silicene having sp 3... | Download Scientific Diagram
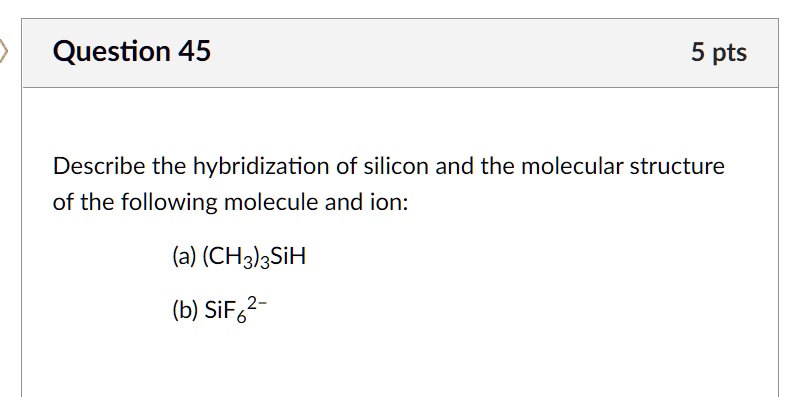
SOLVED: **NOTE: SHOW ALL YOUR WORK AND EXPLANATION. Question 45 5 pts Describe the hybridization of silicon and the molecular structure of the following molecule and ion: (a) (CH3)3SiH (b) SiF2

physical chemistry - What are the height and width of the large and small nodes of the sp3 hybridized orbitals of carbon and silicon? - Chemistry Stack Exchange